Understanding the Gut Autoimmune Connection
What You Need To Know
Author:
Amanda Ledwith, BHSc Naturopathy
Last Updated:
19 Jan 2026
Reading Time:
26 mins
Categories:
Gut Connections
gut-autoimmune-connection
What You'll Learn
Autoimmune conditions occur when the immune system mistakenly attacks the body's own tissues. Research shows gut dysbiosis (microbiome imbalance) plays a leading role in immune dysregulation and autoimmune symptom development.
Common autoimmune conditions linked to gut health:
Lupus (SLE) - restricted microbiome diversity, increased Proteobacteria
Rheumatoid Arthritis (RA) - leaky gut, intestinal permeability
Psoriasis - gut dysbiosis, disrupted anti-inflammatory butyrate pathway
Multiple Sclerosis (MS) - altered gut microbiota, decreased faecal SCFAs
Inflammatory Bowel Disease (IBD) - Crohn's disease, ulcerative colitis
Endometriosis & PCOS - elevated inflammatory LPS markers, gmGUS enzyme
Common gut patterns in autoimmune conditions:
Depleted beneficial bacteria (Bifidobacterium, Faecalibacterium prausnitzii, Lactobacillus, Akkermansia)
Elevated inflammatory bacteria (Proteobacteria, Prevotella, Bacteroides)
Leaky gut/intestinal permeability (allows microbial components into bloodstream)
Reduced SCFA production (particularly butyrate - critical for immune regulation)
Why testing matters:
Identifies specific bacterial imbalances contributing to autoimmune symptoms
Reveals leaky gut markers (LPS, gmGUS enzyme)
Guides targeted protocols (restore beneficial bacteria, reduce inflammatory species, repair gut barrier)
Tracks progress with retesting
Our approach: Restore microbial balance, optimise gut barrier function, modulate immune responses through dietary interventions, targeted supplements, and personalised protocols based oncomprehensive testing.
Gut health is not often the first thing people think of when faced with autoimmune symptoms—but it should be.
Ever wondered why your lupus, rheumatoid arthritis or IBD symptoms settle when you avoid certain foods? Or why flares seem to pop up after stressful events or around Christmas?
Our team has overwhelmingly positive results with many autoimmune conditions, including psoriasis and Crohn's, by testing and then treating the gut.
Research shows that it works because of the strong connection between the gut microbiome and the immune system.
Autoimmune conditions are thought to occur when the immune system mistakenly attacks the body's own tissues, leading to inflammation and tissue damage.
We now know that dysbiosis (gut microbiota imbalance) has a leading role contributing to immune dysregulation and the development or exacerbation of autoimmune symptoms.
Rather than merely treating symptoms, we focus on identifying and addressing underlying imbalances that may be contributing to gut dysfunction and downstream immune dysregulation.
In this article, we'll explore the significance of the autoimmune gut connection and then explore the basic mechanisms behind some of the most common autoimmune conditions.
1. The Gut Microbiome & Autoimmunity
By now you probably have a good idea of what the gut microbiome is exactly, so I won't go too far into all the details—we'll jump straight into the gut-autoimmune connection.
[But if you still aren't sure, read our article How to Improve Gut Health Naturally: The Ultimate Guide to get started.]
Let's start with what we know.
We know the gut microbiome plays a crucial role in regulating the immune system.
It actually helps to train and modulate our immune responses. Meaning it teaches our immune cells how to distinguish between harmful pathogens and beneficial microbes.
For example the beneficial group Bifidobacterium, trains the immune system by promoting the production of anti-inflammatory cytokines, such as interleukin-10 (IL-10), which helps maintain immune balance and dampen excessive inflammatory responses.
Dysbiosis, or imbalance occurs, there's a disruption in the delicate balance of these microbial communities.
This often looks like lower or absent levels of beneficial bacteria (like Bifidobacterium) in gut microbiome test results. And over time leads to compromised immune function and increased susceptibility to overactive inflammatory responses.
We also see this reduced capacity to deal with inflammation as a reduction in Faecalibacterium prausnitzii. Low levels of F. prausnitzii have been associated with inflammatory bowel disease (IBD) like Crohn's and other autoimmune disorders.
Additionally, a healthy gut microbiome contributes to proper nutrient absorption, metabolic regulation. And ultimately supports a regulated immune approach against both bacterial and viral pathogens—some of which like Epstein-Barr Virus (EBV also known as glandular fever) are connected to specific autoimmune conditions.
"After reviewing over 2,000 microbiome test reports alongside Amanda, I see consistent patterns in clients with autoimmune conditions—regardless of which specific condition they have.
Common patterns across lupus, RA, psoriasis, MS, IBD, endometriosis, and PCOS:
1. Severely depleted Faecalibacterium prausnitzii (our primary butyrate producer) This bacteria is critical for gut barrier integrity and immune regulation. When F. prausnitzii is low, I consistently see elevated inflammatory markers and leaky gut indicators. Butyrate production is compromised—which means the gut barrier can't repair itself effectively and inflammatory signals increase systemically.
2. Elevated Proteobacteria (particularly Klebsiella, Proteus, Escherichia) These bacteria produce LPS (lipopolysaccharide)—a potent inflammatory molecule that crosses compromised gut barriers and triggers systemic immune responses. In autoimmune clients, I often see Proteobacteria levels 3–5 times higher than healthy ranges. This drives the chronic inflammation underlying autoimmune flares.
3. Depleted beneficial bacteria (Bifidobacterium, Lactobacillus, Akkermansia) These species produce anti-inflammatory compounds, support gut barrier integrity, and train the immune system to distinguish between harmful pathogens and beneficial microbes. When they're severely depleted—as I consistently see in autoimmune conditions—the immune system loses this regulatory balance.
4. Low diversity (200–400 range vs healthy 600+) Autoimmune clients often have severely restricted microbiome diversity, missing hundreds of bacterial species that produce beneficial metabolites (SCFAs, B vitamins, anti-inflammatory compounds). This restricted diversity is often linked to antibiotic history, chronic stress, or long-term restrictive diets.
5. Functional capacity markers: reduced butyrate production, elevated LPS, depleted SCFA pathways These functional markers show WHAT the microbiome is doing—not just which bacteria are present. In autoimmune clients, I see reduced capacity to produce anti-inflammatory compounds and elevated production of inflammatory metabolites.
The critical insight: autoimmune conditions aren't just 'immune system problems'—they're often driven by gut dysbiosis, leaky gut, and bacterial imbalances that trigger and sustain immune dysregulation. Testing reveals these patterns, and targeted protocols can shift bacterial balance, reduce inflammation, and support immune regulation."
— Victoria, Microbiologist
2. Lupus & the Gut Connection
Lupus is an autoimmune disease characterised by inflammation and symptoms in a number of organ systems, it is also called SLE (systemic lupus erythematosus).
It can be both a challenging condition to diagnose and to manage and wasn't until 2014 that a landmark study connected lupus patients with restricted microbiome diversity.
Studies have indicated alterations in the gut microbiota composition of lupus patients, such as increased Proteobacteria, Bacteroidetes and Actinobacteria with lower Firmicutes. Reductions in beneficial bacteria like Lactobacillus and Bifidobacterium are also seen.
These changes in gut microbial balance contribute to immune dysregulation and systemic inflammation observed in lupus.
Additionally, some gut microbiome metabolites, such as 2-hydroxyisobutyrate has been found in the urine of active SLE patients—which is linked with elevated Faecalibacterium prausnitzii.
Further, some studies have correlated supplementation of Akkermansia muciniphila and Lactobacillus plantarum with regulated immune response in SLE patients.
In our patients, we have found, addressing indicated gut imbalances, reducing dietary sources of inflammation and using targeted supplements can prove helpful in reducing symptoms in many people.
3. Rheumatoid Arthritis (RA) & Gut Health
Rheumatoid arthritis (RA) is a chronic autoimmune condition primarily affecting the joints. This begins with inflammation, manifests as pain, and eventual damage.
RA can also impact systemic health, contributing to fatigue, inflammation in other organs, and an increased risk of cardiovascular disease.
Recent studies have pointed towards a potential link between RA and intestinal permeability, also known as "leaky gut."
Intestinal permeability refers to the integrity of the gut barrier, which regulates the passage of substances from the gut lumen into the bloodstream.
In individuals with RA, disruptions in gut barrier function may allow the passage of microbial components and toxins across the gut barrier—triggering immune responses and systemic inflammation.
While the exact mechanisms are still under investigation, evidence suggests that leaky gut may contribute to the development and progression of RA.
We have found that by focusing on a few core principles we can work to mitigate inflammation and support joint health.
We work on:
Restoring gut microbial balance
Optimising gut barrier function
Modulating immune responses
We also recommend gentle dietary interventions and targeted supplements. These may include limiting potential trigger foods and incorporating anti-inflammatory foods rich in antioxidants and omega-3 fatty acids among others.
Probiotics, prebiotics, and specific nutrients (where indicated) are also an important part of our approach.
4. Psoriasis & the Gut Microbiota
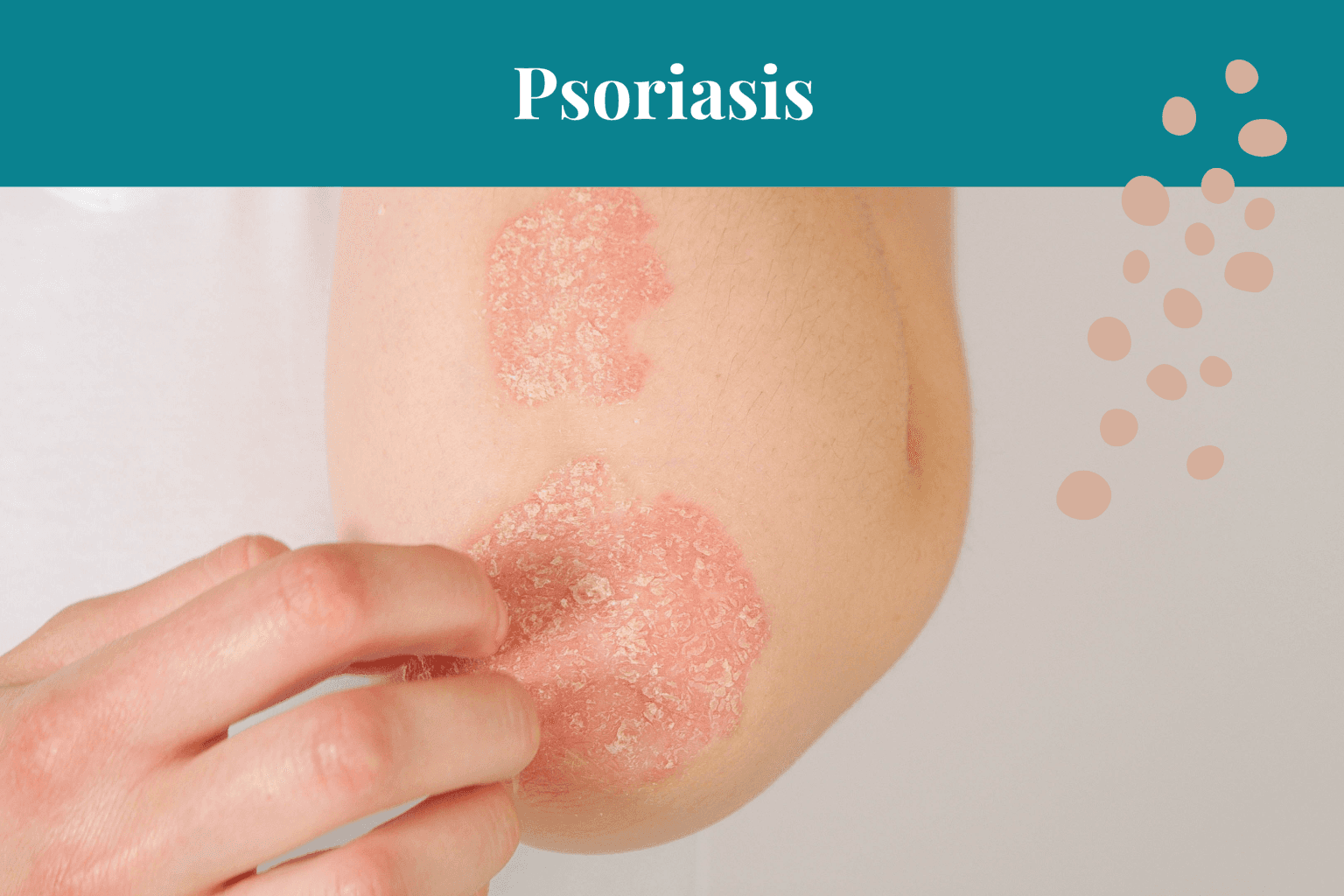
Psoriasis is an inflammatory skin condition characterised by red, inflamed patches of skin with silvery scales—it is now recognised to have autoimmune features.
Beyond its visible manifestations on the skin, psoriasis is associated with systemic inflammation and managing psoriasis can remain a challenge if appropriate attention is not given to healing the gut.
Research has shed light on the association between psoriasis and gut dysbiosis, suggesting that alterations in the gut microbiota composition may contribute to the pathogenesis and exacerbation of psoriasis.
Studies have revealed differences in the gut microbial profile of individuals with psoriasis compared to healthy controls, with reductions in beneficial bacteria like Akkermansia and increases in pro-inflammatory microbes, like Prevotella.
This dysbiosis may disrupt immune homeostasis, trigger systemic inflammation, and influence skin inflammation, but also importantly the anti-inflammatory butyrate pathway.
Amanda has an amazing track record with alleviating psoriasis symptoms by addressing the root cause in the gut.
5. Multiple Sclerosis (MS) & Gut Dysbiosis
Multiple sclerosis (MS) is a neurological autoimmune disease characterised by inflammation and damage to the central nervous system (CNS), including the brain and spinal cord.
This chronic condition can manifest in a variety of symptoms, including fatigue, muscle weakness, numbness, and problems with coordination and balance.
MS occurs when the immune system mistakenly attacks the myelin sheath, the protective covering of nerve fibers, leading to disruptions in nerve signalling and function.
Emerging research has begun to uncover a potential link between MS and alterations in the gut microbiota.
Studies have revealed differences in the composition of gut microbial communities between individuals with MS and those without. Interestingly, increased Akkermansia has been observed in MS patients.
Significantly, a general decrease in faecal SCFA levels are also seen.
All this indicates a very strong connection between the gut microbiome and MS, and offers promise for those suffering that potential gut microbiome changes can improve some outcomes.
6. Inflammatory Bowel Disease (IBD) & the Gut Microbiome
Inflammatory bowel disease (IBD) comprises two main conditions, both of which involve chronic gut inflammation. They are:
Crohn's disease: Crohn's disease is characterised by patchy inflammation that can penetrate deep into the intestinal walls—it can affect any part of the digestive tract, from the mouth to the anus.
Ulcerative colitis: Ulcerative colitis primarily affects the colon and rectum, leading to inflammation and ulceration of the colonic mucosal lining.
Extensive research has established a correlation between IBD and dysregulated gut microbiota. In individuals with IBD, there are often disruptions in the composition and function of the gut microbiome, with alterations in microbial diversity and abundance.
Specific gut microbiome species have been associated with IBD, for example:
Some Bacteroides species such as B. fragilis have been found to bloom before and during pouchitis in UC.
Transient increases in Ruminococcus gnavus have been noted in colitis studies.
Bilophila wadsworthia has been implicated with human IBD and stimulating inflammatory pathways in animal studies.
If you're dealing with Crohn's disease or ulcerative colitis, our IBD Specialist program uses comprehensive testing to identify the specific bacterial imbalances contributing to your symptoms.
7. Endometriosis, PCOS & Gut Health
Endometriosis and polycystic ovary syndrome (PCOS) are female reproductive hormonal disorders that can significantly impact fertility and general wellbeing.
While traditionally viewed as gynaecological conditions, mounting evidence suggests potential autoimmune (and microbiome) components in their pathogenesis. More and more practitioners are advocating dietary changes to help manage and in some cases reverse symptoms.
Additionally, bacterial inflammatory markers such as LPS (lipopolysaccharide) and gut microbial β-glucuronidase (gmGUS) are important inflammatory markers in oestrogen-induced conditions.
Our Gut Microbiome Testing results include these markers.
Book Your Free Evaluation Call
Endometriosis is an inflammatory, oestrogen-dependent condition characterised by the presence of endometrial tissue outside of the uterus. In addition to debilitating gynaecological symptoms, gastrointestinal symptoms are thought to affect up to 90% of sufferers, including bloating followed by nausea, constipation, diarrhoea and vomiting.
Microbiome studies have shown leaky gut indicating gram-negative LPS bacteria to be consistently higher in endometriosis patients, especially so in those with gut symptoms. These include the Gammaproteobacteria which includes pathogenic bacteria such as Escherichia/Shigella, and Bacteroides and Prevotella.
A study published in 2023 has highlighted gmGUS as a potential biomarker for endometriosis. Gut microbial β-glucuronidase (gmGUS) is an enzyme some bacteria including Faecalibacterium prausnitzii and select Bacteroides species produce, it acts to reverse phase II liver detoxification and negatively impacts oestrogen recycling in the body.
Similarly, in PCOS, many microbiome connections have been identified, particularly changes also associated with insulin resistance, chronic inflammation and metabolic syndrome.
Gut bacteria associated with a high-fat, high-sugar diet including Enterobacteriaceae and Prevotella have been associated with insulin-resistant PCOS.
Furthermore, inflammatory leaky gut indicators like LPS seen in endometriosis are also present in PCOS.
Research is continuing but the connection is becoming clear that diet and therefore microbiome play a significant role in disease progression for both endometriosis and PCOS.

